Abstract
Purpose
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin's lymphoma. The association of rituximab to CHOP chemotherapy is nowadays the standard of care for de novo DLBCL patients; however 40% of these patients relapse or are refractory to treatment within 2 years. Before rituximab, patients treated by high dose chemotherapy plus autologous stem-cell support (HDT) had a significantly better outcome than by CHOP alone 1. Between 2005 and 2008, the GOELAMS 075 French multicentric clinical trial (NCT00561379) enrolled 323 de novo DLBCL patients randomly treated by R-CHOP or R-HDT, and showed that rituximab erases the survival difference between the two arms of frontline treatment 2.
The main objective of this study was to evaluate circulating tumor DNA (ctDNA) concentration measured by an easy-to-collect and cost-effective method in the GOELAMS 075 de novo DLBCL patients, in regards of the two frontline treatments.
Methods
Plasma samples were collected at inclusion for 123 patients and 6 months after the end-of-treatment (EOT) for 50 patients in complete remission (CR) at EOT. The 123 patients were representative of the 323 eligible patients. Among the 123 patients, 68 were assigned to the R-CHOP arm and 55 to the R-HDT arm, of which, respectively, 59 and 51 achieved CR at EOT. Baseline characteristics, overall (OS) and progression free survivals were not different between randomization arms for the 123 patients. No difference in baseline characteristics was found between both studied cohorts.
ctDNA concentration in ng per ml of plasma was measured as the enrichment of DNA fragments between 100 and 300 base pairs. For survival analysis, the ctDNA threshold was defined as equal to 10 times the maximum cell-free DNA concentration measured in healthy subjects. For paired samples, mutation profiles at baseline and 6 months after EOT were assessed by capture-based targeted DNA sequencing using a panel of 43 genes known to be associated to DLBCL in the literature. Intention-to-treat (ITT) analysis was performed, unless otherwise mentioned.
Results
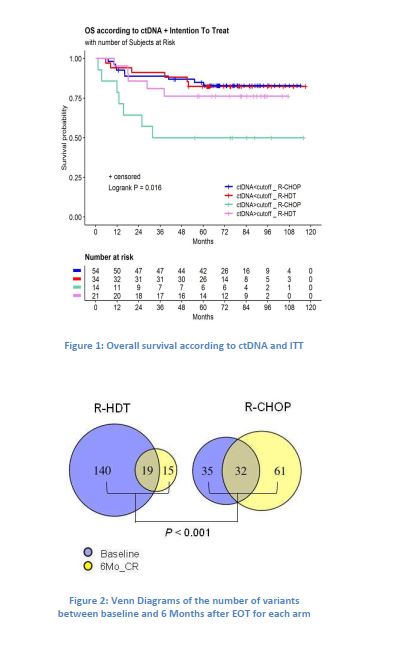
Elevated ctDNA at diagnosis was found significantly associated with adverse clinical factors (R-IPI ≥3 ; number of extranodal sites ≥2), as well as intake of salvage therapy. Patients with ctDNA ≥ 54.9 ng/ml had significantly worse OS (HR=2.4, 95 th CI : 1.1-5.2 ; Pvalue=0.029). Patients with elevated ctDNA were more likely to require a salvage therapy, regardless of the randomisation arm. Only for ITT R-CHOP patients, elevated ctDNA was associated with a significantly shorter OS (HR=4.4, 95th CI : 1.6-12.3 ; Pvalue=0.004; Figure 1). Patients with elevated ctDNA who received the initially planned 8 R-CHOP cures (no salvage therapy) had a significantly worse OS than any other patients (67% deaths ; 5-years OS : 33% ; Pvalue=0.0002).
Focusing on the cohort of 50 patients in CR at the EOT, we found a significant decrease of ctDNA 6 months after EOT compared to baseline only for ITT R-HDT patients (Pvalue=0.006). Noticeably, out of the 7 patients with higher ctDNA 6 months after EOT, six had been assigned to the R-CHOP arm, and five of those did not benefit salvage therapy. Mutation profiling was performed for 46 CR patients (ITT, R-CHOP, n=23 ; R-HDT, n=23) at baseline and 6 months after EOT. Mutations were detected in 48% of R-CHOP patients and 61% of R-HDT patients. The tumor mutational burden, defined as the number of gene mutations found on the total of 164 Kb sequenced, was significantly decreased 6 months after EOT only for R-HDT patients (Pvalue=0.005). The number of mutations, especially the number of new variants, and the number of deleterious and likely oncogenic variants, increased significantly in the R-CHOP arm 6 months after EOT while decreasing in the R-HDT arm (Pvalue<0.001; Figure 2). All 46 patients in CR at EOT remained in CR 5 to 10 years after treatment, except for 3 patients, who did not receive salvage therapy and relapsed more than 5 years after EOT. All 3 patients belonged to the R-CHOP arm patients with higher ctDNA 6 months after EOT compared to baseline.
Conclusion
Using an easy-to-measure and low-cost method, ctDNA concentration at diagnosis was shown, in an intention-to-treat analysis, to be an adverse prognostic factor only for de novo DLBCL patients treated by standard R-CHOP. Furthermore ctDNA was not significantly cleared in plasma at CR only for ITT R-CHOP patients, with the number of mutations increased 6 months after EOT compared to baseline.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal